The focus of our research is on understanding how the hepatic lipid metabolism is controlled and how these mechanisms can be explored for the treatment of obesity-related metabolic diseases. In obesity, hepatic lipid metabolism is altered, leading to the accumulation of triglycerides within the liver and to a spectrum of liver pathologies known as non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most prevalent chronic liver disease worldwide (~25% of the adult population) and is a risk factor for type 2 diabetes, dyslipidemia, and cardiovascular disease. Currently, there is no approved treatment for NAFLD apart from changes in lifestyle. Advances in understanding the molecular mechanisms leading to intrahepatic lipid accumulation are critical to the development of targeted therapies.
Our current studies are divided into two major research lines:
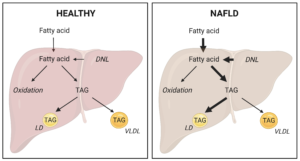
1. Control of the metabolic fate of fatty acids in normal physiology and NAFLD: the goal of this research is to uncover hepatic mechanisms controlling the partitioning of fatty acids into specific downstream metabolic pathways, with focus on triglyceride synthesis vs fatty acid oxidation and intrahepatic storage vs lipoprotein (VLDL) secretion. We aim to manipulate these pathways in order to reduce lipogenesis and increase lipid disposal, thereby reducing hepatic steatosis and mitigating NAFLD.
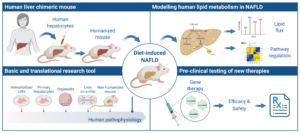
2. Liver-targeted gene editing & humanized mouse for disease modeling and pre-clinical testing of NAFLD therapies: in this unique research approach, we combine human liver chimeric mouse and liver-targeted genome engineering to develop a platform to accelerate the study of liver disorders and the evaluation of experimental therapies in vivo in a human-like setting. We aim to build a bench-to- bedside approach, from basic discovery to improved patient care.